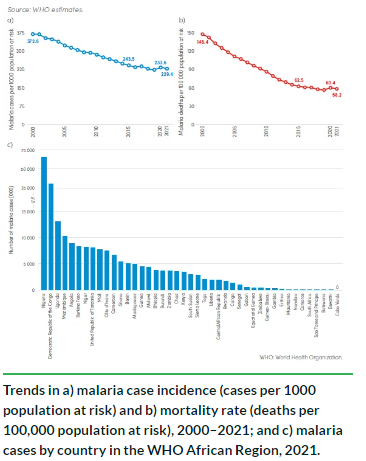
OUAGADOUGOU — Despite being a preventable and treatable disease, malaria is currently affecting the lives of more than 200 million people. This results in over half a million deaths per year, with 80 percent of this mortality occurring in children under the age of 5.
In addition to the tragic social and humanitarian considerations, the economic losses to Africa attributed to malaria equate to around $12 billion a year. Hence, the public health burden of malaria is huge, and continued efforts in malaria control, elimination strategies, and case management are crucial to minimizing the devastation that malaria has on at-risk communities. In this article, we explore the innovative use of genetically modified mosquitoes as a means of fighting this devastating disease.
Dr. Federica Bernardini is a Research Associate at Imperial College London, working on novel strategies to control the mosquito vector of human malaria. Bernardini is a part of Target Malaria, an innovative project that aims to reduce the population of malaria-transmitting mosquitoes in sub-Saharan Africa, where 98 percent of malaria deaths occur. By reducing the population of malaria mosquitoes, the project aims to reduce the transmission of the disease. This not-for-profit consortium consists of a multidisciplinary team of over 200 people in 8 countries. Professions range from protein engineers, molecular biologists, medical entomologists, population biologists, and social scientists, to specialists in risk and regulation, and community engagement advisors.
Bernardini presented a talk at the SelectScience® Virtual Microbiology and Infectious Disease Summit 2022, now available to view on demand, detailing how Target Malaria is exploring using genetically engineered mosquitoes to tackle malaria. This article summarizes the presentation and explores the ongoing effort to develop synthetic biology approaches for the genetic control of malaria-transmitting mosquitoes.
The need for novel disease management strategies for malaria
Malaria is a life-threatening disease caused by a single-cell parasite called Plasmodium, spread to people through the bites of infected female mosquitoes. Male mosquitoes do not bite humans, and therefore do not transmit malaria. There are currently several methods used to reduce malaria infection, such as mosquito spray, insecticides, bed nets, and antimalarial drugs. These have been implemented for decades and have been very successful in limiting the incidence of malaria and have undoubtedly saved countless lives. Between 2000 and 2015, malaria infections and deaths decreased by 37 percent and 60 percent respectively. However, it has been reported that, since 2015, this progress has plateaued.
Unfortunately, there has been an increase in incidence of resistance to both insecticides and antimalarial drugs. This threat continues to grow, particularly in Africa; 80 percent of the African countries that monitor this trend have reported resistance to at least one insecticide. This resistance is alarming, and perhaps contributes to the plateau in the decrease of progress seen against malaria. Thus, novel prevention strategies are required to tackle this disease. Target Malaria is one such project and has been pioneering technological innovations in the field of gene editing in the context of malaria disease management.
Target Malaria’s objectives
- To develop and share a novel genetic technology for vector control of Anopheles mosquitoes to contribute to reducing the burden of malaria in Africa
- To create a path for responsible research and development of genetic technologies
- To use an approach which is complementary to existing methods
Mosquito genetics 101: Mendelian inheritance
The ability of mosquitoes to transmit malaria is genetically determined. Factors such as longevity, feeding behavior, parasite development, and reproduction are all important elements that influence the likelihood of a mosquito to transmit malaria to a human. For example, the longer a mosquito lives, the higher the chance that mosquito has of biting a human and transmitting malaria. The more mosquitoes reproduce, the more mosquitoes there are, thus increasing the probability of transmission. The rationale behind the Target Malaria approach is that if it is true that these features are genetically determined, then perhaps the genes that are responsible for these features can be modified to impair the vectoral capacity of mosquitoes.
Due to the medical significance of mosquitoes, the insects have been the focus of several genetics studies and there is a relatively good understanding of the mosquito genome.8 Moreover, gene editing technologies have seen huge advancements in recent years, and the dawn of CRISPR-Cas9 technologies has warranted the use of highly efficient and specific targeted gene editing in almost any lab due to its simplicity, cost, flexibility, and ease of access. Bernardini’s lab at Imperial College London, has been harnessing this knowledge along with these newfound molecular tools to develop strategies against malaria.
The team has had good success at genetic engineering and modifying the genome of mosquitoes over the past few years. However, for this approach to translate into a reduction of malaria infections and mortality, the team must be able to modify the genome of these organisms, and spread this genetic modification from just a few individuals to a much larger population in the wild.
Mosquitoes, like humans, have two copies of each chromosome. According to the laws of Mendelian inheritance, the gametes, or reproductive cells (ova or sperm), of genetically engineered mosquitoes with one edited chromosome would have a 50 percent probability of carrying the genetically modified chromosome. The genetic material of a gamete determines which inherited traits will be passed on to the next generation. If such genetically engineered mosquitoes were released in the wild, as mosquitoes found in the wild (wildtype) do not carry the transgene, there is a 50 percent chance that the progeny of a wildtype and transgenic mosquito would carry the desired transgene. Therefore, statistically, the frequency of a genetic modification would not increase above its release frequency. This means that the altered gene would not spread in the wild as needed for it to have an impact on the malaria disease burden.
To tackle malaria, Bernardini and her team must overcome this challenge, and spread a genetic modification from a few individuals, to a large population of mosquitoes and spread this mutation at a faster speed. To do this, they have been using an approach, based on genetic engineering technology, known as a gene drive.
Gene drive: The driving force behind the genetic control of malaria-transmitting mosquitoes
A gene drive is a genetic phenomenon that occurs in nature and causes a genetic trait to rapidly spread in a species from generation to generation. Normally, a genetic trait has a 50 percent chance to be passed on to the offspring. In the presence of a gene drive this chance increases to up to 99 percent. If this mechanism is applied in the reproductive organs of our mosquitoes, this would increase the chance of inheritance for the gene of interest. This phenomenon is known as homing.
The coupling of this homing mechanism to a genetic modification would allow this modification to be copied in the homologous chromosome. This facilitates the potential to overcome Mendelian rules of segregation, enabling the modification to be inherited by all progeny. As a result, this modification could quickly increase in the target population. Using this mechanism, in theory, Target Malaria would be able to spread a genetically engineered trait through a mosquito population very quickly.
Selfish genetic element mechanism: Explained
An example of a selfish genetic element is homing endonucleases. These recognize a specific site in the genome of an organism in a sequence-specific manner. As mosquitoes have two copies of each chromosome, there will be two recognition sites. The homing endonuclease gene integrates into one insertion site. When the homing endonuclease protein is produced, the endonuclease creates a cut in the DNA at the recognition site within the matching chromosome. This triggers the cell’s DNA repair machinery, which requires a template DNA strand to copy when fixing the break. The repair machinery uses the uncut homologous chromosome as a template. This chromosome contains the selfish genetic element at the specific site of the cut on the other chromosome. Therefore, the template contains the homing endonuclease gene, resulting in its integration into the second chromosome. This process results in the conversion of a heterozygote into a homozygote individual.
Using genetically engineered mosquitoes to suppress population
Now we have this mechanism of increased transmission, we must consider the genetic modification that we want to spread. There are several features that could be targeted to impair the vectorial capacity of the mosquitoes. As an example, lets focus on reproduction. Disrupting a gene that is important for female fertility would target reproduction as this will generate female mosquitoes that are sterile.
This seems like a paradoxical concept. How can infertility be spread through a population? The answer to this is simple. Transgenic males carrying one or two copies of this modification can continue to mate and spread the gene to offspring. When females are heterozygous for the mutation, carrying just one copy, they are still able to reproduce and pass on the gene to progeny. The homozygous female mosquitoes that carry the genetic modification in both chromosomes are sterile and are not able to produce progeny.
So, if these transgenic mosquitoes are released into the wild, heterozygous females, heterozygous males, and homozygous males, can mate and produce progeny. As Bernardini explains in her summit presentation, eventually, the number of transgenic mosquitoes in the wild would increase to the point that progeny that have both copies of the modification will be produced, female mosquitoes will be sterile, and this would then lead to a reduction in the targeted mosquito population.
Target Malaria have implemented this approach using CRISPR-Cas9 gene editing. CRISPR-Cas9 acts as a selfish genetic element that can trigger super Mendelian inheritance of a genetic modification (>50%).14 This tool acts as molecular scissors, facilitating the targeted induction of modifications into the genome of an organism. In fact, when expressed in the right place, i.e. the reproductive organs of these mosquitoes, and at the right time, these CRISPR-Cas9 tools can lead to the same homing process described for endonuclease genes.
The team identified genes important for female fertility and introduced the CRISPR-Cas9 tools within their sequence. The insertion of this genetic element within the female fertility gene disrupts the function of the gene. The presence of these elements not only disrupts the function of the fertility gene, but, in the reproductive organs of the mosquito, it also allows this modification to be copied in the matching homologous chromosome through the process of homing. This means that Target Malaria can disrupt genes that are important for fertility and can also ensure that this genetic mutation can propagate and spread through the generations thanks to the gene drive mechanism that is coupled with the mutation. Indeed, Target Malaria’s team of researchers have been successful in generating transgenic mosquitoes with this approach in which the genetic modification is affecting a gene important for female fertility, known as doublesex.
Investigating the efficacy of introducing the transgene into a wildtype mosquito population
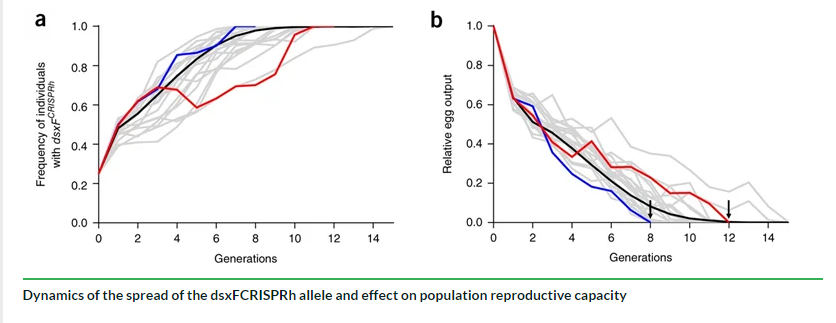
So, Target Malaria has successfully generated these genetically modified mosquitoes conferring female sterility, but what happens if this approach is actualized within a population of mosquitoes? To test this, the team carried out preliminary experiments in which these transgenic mosquitoes were introduced into a caged population of wildtype mosquitoes in a laboratory setting. In her talk, Bernardini explains that the experiment consisted of four cages, with a population size of about 600 mosquitoes per cage. The aim of this investigation was to ask: If we introduce these transgenic mosquitoes into the caged population, is the genetic modification able to spread and cause suppression of the mosquito population by generation of sterile females?
The mosquito populations were monitored for several months. It was found that the frequency of the genetic modification quickly increased in all cages. The more the genetic modification increased within the population, the more females that were homozygous for the mutation there were, with up to 100% prevalence of the transgene within 7–11 generations. The team observed that the number of eggs produced by mosquitoes in the cages started to decrease to the point that at generations between 8 and 12, the mosquito population collapsed because there were no females left that were fertile and able to produce progeny.
Following the success of this investigation, Bernardini explains that a similar experiment was performed at a larger scale, in large cages, where this gene-drive technology can be challenged with vector ecology. These trials allow the team to monitor the dynamics of the gene drive in aged-structured populations in presence of complex feeding and reproductive behaviours. This aimed to provide a setting that better mimics a more complex environment such as that in the wild. For instance, Bernardini explains, mosquitoes in these large cages can make swarms which are important for the mating behaviour of this organism. There are also ways to mimic the sunset, for example by using specific lights.
Interestingly, even within this larger population, the transgene very quickly increased in frequency due to the gene drive coupled with it. As hoped, the number of eggs decreased to the point that no more eggs were produced due to the appearance of female sterility. These investigations indicate that the approach has the potential to be a viable solution for mosquito population suppression in the wild.
Beyond the lab, the Target Malaria team have carried out a study in which non gene drive genetically modified mosquitoes have been released into the wild. The study aimed to collect data on the dynamics of transgene-carrying mosquitoes in a real-world scenario including survival and dispersal. The results provided valuable information on the behavior and fitness of released genetically modified mosquitoes that will inform future release operations.
Concluding remarks
Target Malaria seems to be edging ever closer towards its ultimate goal of reducing the transmission of malaria through the population suppression of malaria-transmitting mosquitoes in sub-Saharan Africa. The scientific innovation behind this approach is a compelling insight into the potential applications of gene editing technology. Translating this concept to a reduction of malaria-transmitting mosquitoes is a complex challenge, with geographical, operational, political, and ethical considerations. With a continued effort of the multidisciplinary team at Target Malaria, the project may hold promise to work alongside existing and other novel disease management strategies, to reduce the devastating consequences of malaria in sub-Saharan Africa.
https://thescientistschannel.com/federica-bernardini


