Around the world there are some 463 million adults, or roughly one in 11, who suffer from the genetic type -1 (a minority of diabetes) and from type-2 that is caused by overweight, lack of exercise, a sedentary lifestyle, urbanization, poor diets, sedentary lifestyles, overprocessed foods and poor diets. By 2045, over 700 million could be afflicted, posing overwhelming challenges to healthcare, economies, and public health efforts.
Healthcare systems agree that urgent action is needed to stem this tide, via effective prevention strategies, better access to care, and innovative treatments.
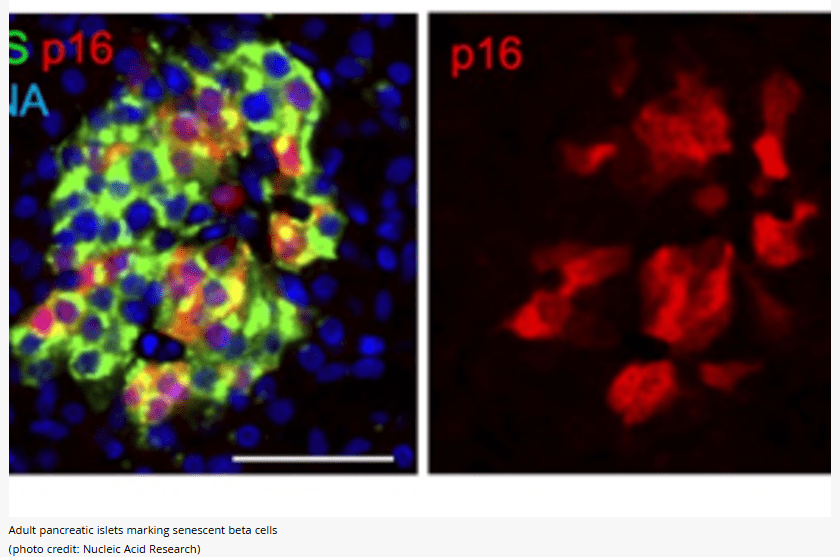
The main role of adult human pancreatic beta cells is to activate a gene called p16 that means they are in an aging-like state called cellular senescence. Instead of showing signs of dysfunctionality, these cells present high levels of genes that are vital for their function so they appear to have a higher level of functionality and maturity compared to their non-aged neighbors. This is surprising because previously identified senescent cells in other tissues are usually regarded as dysfunctional and having harmful effects.
By analyzing the gene organization of senescent beta cells, the researchers discovered that they change the packaging of the genes – the chromatin, generating a reprogrammed organization that allows activation of functionality. Because of this, it seems that the aging beta cells have the ability to release insulin in response to glucose in even larger amounts, which helps regulate blood sugar levels effectively.
The team also found that senescent beta cells have elevated levels of genes that communicate with the immune system. This response called the “interferon response,” normally reacts to a viral infection, calling on immune cells to attack the invader, but the senescence beta cells activate this pathway in the absence of such infection: it is molecular changes in the cells themselves simulate this response. The potential consequence is increased stimulation of immune cells to attack beta cells, the fundamental process that drives type I diabetes.
This means that aging beta cells could help regulate immune responses and be important for understanding autoimmune reactions in type-1 diabetes. Potentially, blocking this response or the process of senescence, could be used to prevent the progression of type-I diabetes in its early stages.
The discovery that aging pancreatic beta cells can remain very functional and respond to immune signals counters the traditional view that senescent cells are purely detrimental. This new understanding opens the door to potential therapies aimed at preserving or enhancing the insulin-secreting function of beta cells in diabetic patients.
“These key findings suggest that senescent beta cells are not a liability, but may act, in a pre-designed manner, to improve insulin production as we age, countering other detrimental effects,” said Ben Porath. “If it is proven in the future that senescence of beta cells is a prominent feature of the early stages of type-1 diabetes, targeting of these cells through drug treatment could represent a novel approach for preventing autoimmune attack of beta cells.”
The team plan in the future to dig deeper into the mechanisms driving the increased activity of functional-maturation programs in aging beta cells, influenced by chromatin dynamics. A comprehensive understanding of these processes holds promise for the development of targeted therapies aimed at enhancing beta-cell functionality and lifespan and improve the quality of life for type-1 diabetes patients, understand how senescence affects the interaction of immune cells with beta cells, and whether this is indeed associated with diabetes, may open the door for new treatment approaches.