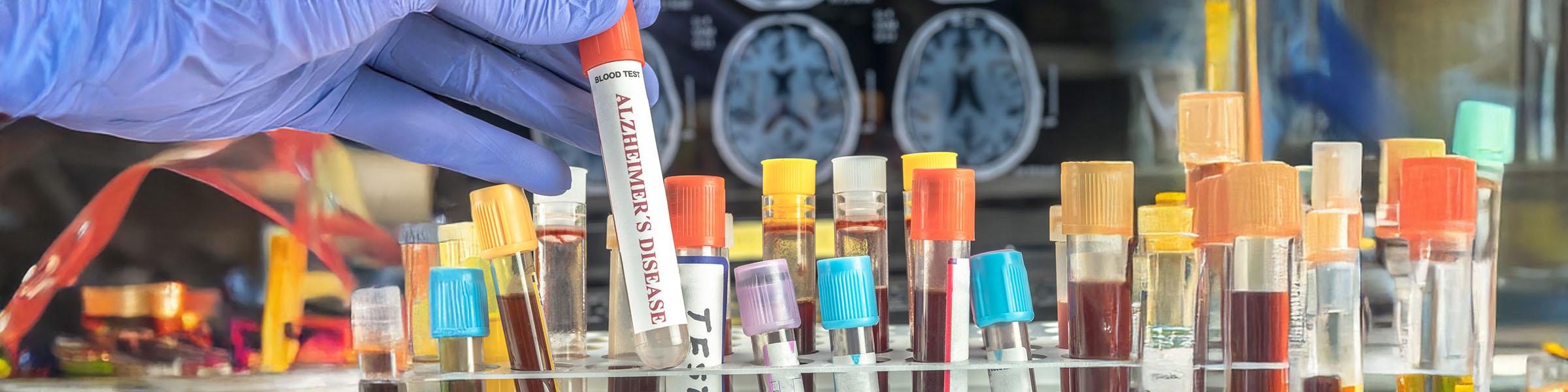
Alzheimer’s disease is the most common form of dementia. For millions of Europeans – and the health services that care for them – it is a ticking time bomb, with still no cure. But EU-funded researchers are developing a simple tool to enable much earlier detection, potentially decades before symptoms appear.
Early detection matters because treatment is most effective when started as soon as possible. This gives people a better chance to slow the progression of the disease and plan for the future. Today, around 7 million people in Europe live with Alzheimer’s, a number expected to double by 2030, according to the European Brain Council.
For Dr Aristeidis Bakandritsos, the challenge is clear: if Alzheimer’s disease is to be detected early enough to make a real difference, testing must become simpler, cheaper and far less invasive.
“Early detection will only be realistic for people when it’s inexpensive for the health service, and painless and simple for the patient, which it isn’t today,” said Bakandritsos, a senior researcher at the Czech Advanced Technology and Research Institute (CATRIN), part of Palacký University Olomouc in Czechia.
Towards routine screening
“
Early detection will only be realistic for people when it’s inexpensive for the health service, and painless and simple for the patient, which it isn’t today.
Bakandritsos is coordinating a four-year EU-funded research initiative launched in October 2023 to transform how Alzheimer’s disease is detected. Called 2D-BioPAD, the project is developing an affordable device capable of identifying up to five Alzheimer’s-related proteins – known as biomarkers – from a simple blood sample.
The aim is not to replace existing diagnostic methods such as brain scans or spinal taps, but to enable much earlier screening in routine healthcare settings. By flagging people at risk before symptoms appear, doctors could intervene sooner, when treatments are most effective.
Clinical pilot studies are now under way in Finland, Greece and Germany to assess how well the tool performs, as well as its safety, ethical implications and how it could fit into routine healthcare.
From invasive testing to a simple blood sample
Diagnosing Alzheimer’s remains complex and often invasive. Today, confirmation typically relies on brain imaging or spinal taps to analyse cerebrospinal fluid – a liquid found around the brain and spinal cord to keep them safe. While blood-based tests are starting to emerge, they are still largely confined to specialist memory clinics.
The 2D-BioPAD team aims to change that. Their vision is a blood test that is faster, cheaper and far less invasive – one that could eventually be used in everyday healthcare settings.
If successful, family doctors could flag people at risk much earlier, long before memory loss and confusion become obvious.
Early treatment is key
Until recently, treatment options focused mainly on managing symptoms. That changed in 2025, when the EU approved the first two disease-modifying Alzheimer’s therapies.
These drugs target amyloid-beta plaques, protein fragments that build up between brain cells and interfere with communication. More importantly, they work best in the early stages of the disease, making timely diagnosis more crucial than ever.
Vincent Bouchiat, CEO and co-founder of Grapheal SAS, a project partner specialising in graphene-based healthcare technologies, acknowledges the ethical complexity of early detection, but points to the advantages.
“There are good reasons for knowing you may go on to develop dementia. New Alzheimer’s medications show promise in delaying the progression of the disease, which is obviously a huge step forward.”
How graphene enables smarter testing
At the core of the 2D-BioPAD device is graphene – a one-atom-thick sheet of carbon that is stronger than a same sheet made of steel and an excellent conductor of electricity.
When Alzheimer’s-related proteins bind to the graphene surface, they subtly change how electricity flows through the material. These changes can in principle be detected with extraordinary sensitivity, motivating researchers to harness this possibility and identify biomarkers present in extremely low concentrations.
Compared with laboratory-based blood tests now entering the market, the envisioned device can offer several advantages: results in around 30 minutes, testing in primary care settings, detection of multiple biomarkers at once and significantly lower costs.
The project brings together 11 partners from 8 European countries, combining expertise in nanotechnology, clinical research and digital innovation.
By the end of 2026, researchers expect to know how the technology performs compared with existing lab equipment.
If results are positive, the team hopes to secure further funding to move towards commercialisation through additional trials, validation and regulatory approvals. The researchers are hopeful the test could be in regular use in as little as five years.
Bringing testing into everyday care
“
We hope this shift will ultimately benefit not only people in the early stages of Alzheimer’s, but also people with no known cognitive problems.
The long-term vision is simple, portable screening that fits easily into everyday healthcare. A small blood sample similar to a diabetes test would be inserted into a compact device connected to a tablet or smartphone.
Rather than a yes-or-no answer, doctors would receive the concentration of relevant biomarkers, helping them decide whether further testing is needed. Used this way, the device would act as an early warning system, not a standalone diagnosis.
The test works a little like the rapid lateral-flow tests familiar from the COVID-19 pandemic, but with far greater analytical power. Measuring several biomarkers at once significantly improves reliability compared with single-marker tests.
AI plays a key role, too. AI and machine learning help design the tiny molecular “probes” that detect Alzheimer’s proteins, optimising them through rapid simulations based on protein databases and molecular models.
Affordable solution to a global problem
Cost is a major barrier to widespread screening. Today’s laboratory-based tests can cost €40 or more per biomarker, while the required equipment can reach hundreds of thousands of euros.
The 2D-BioPAD system aims to reduce that dramatically, combining a low-cost detection device with disposable cartridges that test multiple biomarkers at once.
Jean Georges, executive director of Alzheimer Europe, a pan-European network of national dementia associations, sees this as part of a broader shift from focusing solely on treatment towards improving early detection and reducing risk.
“We hope this shift will ultimately benefit not only people in the early stages of Alzheimer’s, but also people with no known cognitive problems,” he said.
At the same time, he stresses the need for honesty and compassion. No test can predict exactly who will develop dementia or how it will progress.
“Dementia risk prediction comes with ethical, legal and social implications,” Georges said. “Disclosure should always be approached in an open, honest, empathetic and compassionate manner.”
Research in this article was funded by the EU’s Horizon Programme. The views of the interviewees don’t necessarily reflect those of the European Commission. If you liked this article, please consider sharing it on social media.