Every year, millions of lives are suddenly, swiftly transformed by a stroke, which occurs when a blood vessel travelling towards the brain becomes obstructed, causing neurons to die off. Strokes are among the leading causes of disability in adults, and it is estimated that one in six people will suffer one at some point in their lives.
The human brain is by far the most complex organ in our bodies. Its sophisticated cellular architecture and neural networks give us language, memory and abstract reasoning. But this complexity comes at a cost, as brain tissue has a very limited ability to regenerate. Unlike skin or liver tissue, neurons that die are rarely replaced.
This is why brain injuries are the root cause of so many age-related diseases. One of the most serious and common of these is ischaemic stroke, caused by the interruption of blood flow to an area of the brain. Although advances in emergency treatment have improved survival rates, there is currently no therapy capable of repairing the neuronal damage caused by a stroke.
Santiago Ramos
Rehabilitation helps restore some function, but in many cases stroke survivors live with permanent motor and cognitive impairments, as well as an increased risk of depression, dementia and other neurodegenerative diseases. However, this could soon change thanks to the development of stem cell-based therapies.
Read more:
From contraception to menopause: why women face a higher risk of stroke
A new therapeutic horizon
In recent decades, cell therapies have opened the door to a new generation of treatments in regenerative medicine. These are therapies that seek to replace or repair damaged tissue by introducing new cells capable of surviving, maturing and ultimately performing the functions that have been lost.
This is especially important for conditions that affect the brain. Despite its high potential, regenerative medicine has developed relatively slowly because it needs to comply with legislation in different regions. It also requires large financial investments.
A crucial precedent took place in the late 1980s at Lund University Hospital in Sweden, where a team led by Anders Björklund and Olle Lindvall successfully transplanted neural stem cells into the brains of patients with Parkinson’s disease. Parkinson’s is a neurodegenerative disorder characterised by the progressive loss of dopaminergic neurons, which are essential for controlling body movement.
The results were extraordinary: by replacing damaged neurons, many patients regained motor function for more than a decade. These experiments provided the first solid evidence that the human brain can be repaired using living cells.
Olle Lindvall.
Since then, research has advanced, techniques have been refined, and European regulations have established strict frameworks to ensure the safety and quality of these treatments, which are now classed as advanced therapy medicinal products. Currently, various clinical trials are underway around the world that continue Björklund and Lindvall’s work, and offer hope to patients with Parkinson’s and many other diseases that affect the brain.
The unique challenge of strokes
Although this story inspired many studies, strokes present a different challenge to Parkinson’s disease. Ischemic damage is usually more extensive. It also does not affect just one cell type, but multiple populations of neurons, glial cells and blood vessels.
Furthermore, it is not enough for transplanted cells to merely survive in the patient’s brain – they have to integrate functionally. This means they need to send out their axons (the extensions that transmit nerve impulses) and establish synapses or appropriate connections with the surviving neurons, becoming part of the brain circuits.
It is comparable to rebuilding both a collapsed bridge and the traffic that crosses it: connections must be established in the right way for information to flow. Therefore, in addition to adding new cells, the challenge of strokes is to effectively reconnect the brain.
The promise of genetic engineering
This is where genetic engineering, one of the most transformative technologies in modern biology, comes into play. This discipline allows cells to be modified to make them more effective, more resistant, or better able to integrate into damaged tissue.
In our case, we have incorporated into the transplanted cells the gene that encodes the BDNF (Brain-Derived Neurotrophic Factor) protein, a neurotrophic factor that aids brain development and promotes axon growth and synapse formation. The aim is to facilitate the functional integration of new neurons into the injured brain, a key step in ensuring that the transplant not only fills a gap, but also restores neuronal communication.
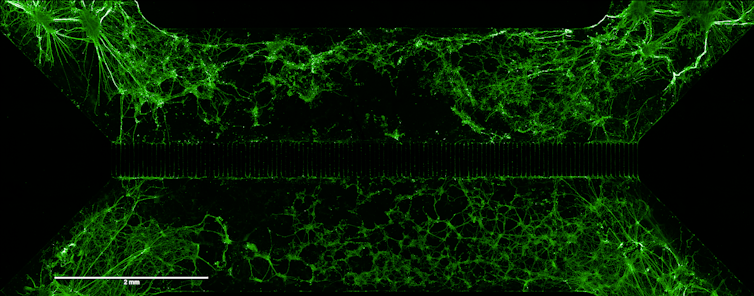
Adapted from IJMS.
A question of ethics
Genetic manipulation also raises ethical dilemmas, especially regarding the limits of its application and its possible long-term effects. The aforementioned transplants in Parkinson’s patients, for instance, were performed with cells from foetal tissue.
Today, thanks to the work of Japanese researcher Shinya Yamanaka, winner of the 2012 Nobel Prize in Medicine, and his discovery of induced pluripotent stem cells (iPS), it is possible to generate stem cells from the patient’s own adult cells. It is now very common to generate iPS cells in a laboratory from skin biopsies.
This avoids many of the ethical conflicts associated with the use of embryos, and reduces the risk of immune rejection. Therefore, the question is no longer whether we can modify cells to repair the brain, but rather what criteria to use, under what regulations, and with what responsibility.
The history of medicine is made up of small victories against the impossible. Just a few decades ago, the idea of healing a stroke-damaged brain would have seemed completely unthinkable. Today, thanks to the combination of biology, genetic engineering and regenerative medicine, it is beginning to take shape in laboratories. Many challenges are yet to be solved, but each new advance reminds us of something essential: not only can the brain learn, it can also be repaired.



