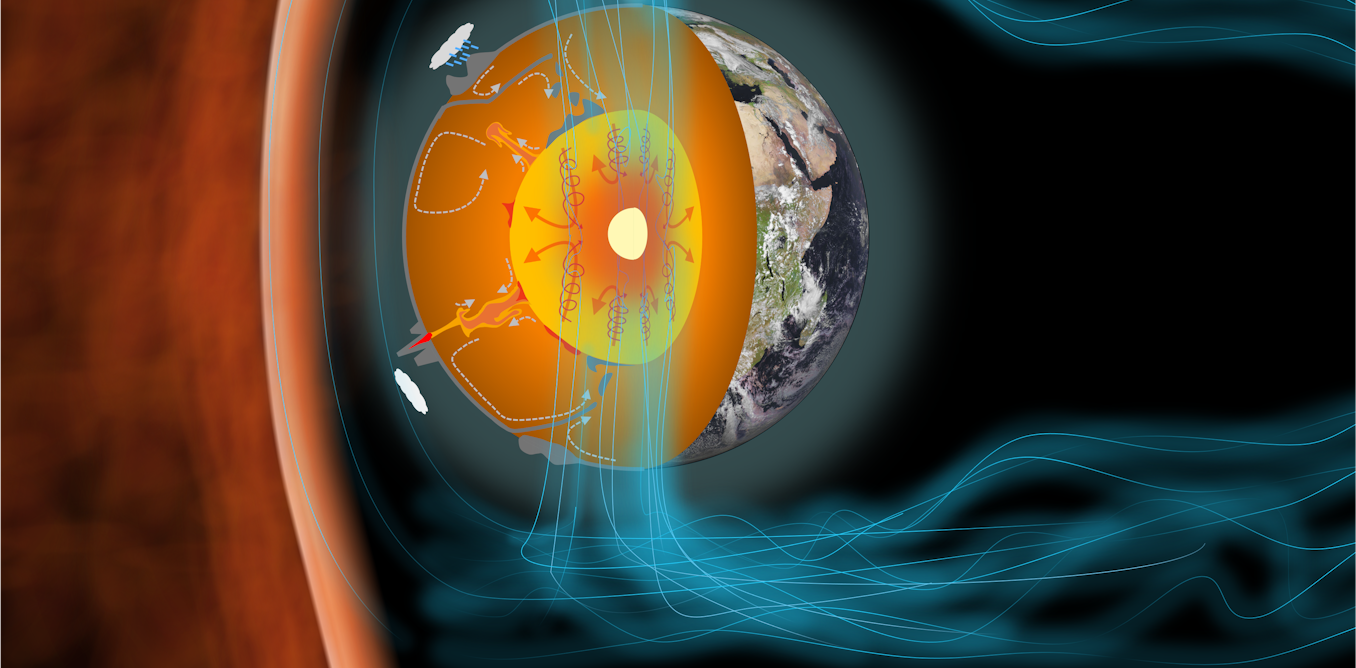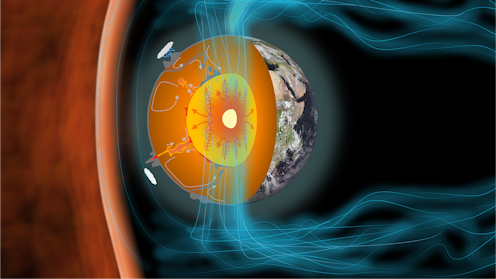
The iron-rich core at the centre of our planet has been a crucial part of Earth’s evolution. The core not only powers the magnetic field which shields our atmosphere and oceans from solar radiation, it also influences plate tectonics which have continually reshaped the continents.
But despite its importance, many of the most fundamental properties of the core are unknown. We do not know exactly how hot the core is, what it is made of or when it began to freeze. Fortunately, a recent discovery by me and my colleagues brings us much closer to answering all three of these mysteries.
We know the temperature of Earth’s inner core is very roughly about 5,000 Kelvin (K) (4,727°C). It was once liquid, but has cooled and become solid over time, expanding outwards in the process. As it cools, it releases heat to the overlying mantle, driving the currents behind plate tectonics.
This same cooling also generates the Earth’s magnetic field. Most of the field’s energy today comes from freezing the liquid part of the core and growing the solid inner core at its centre.
However, because we cannot access the core, we have to estimate its properties to understand how it is cooling.
A key part of understanding the core is knowing its melting temperature. We know where the boundary between the solid inner core and liquid outer core is from seismology (the study of earthquakes). The temperature of the core must equal its melting temperature at this location, because this is where it is freezing. So, if we know what the melting temperature is exactly, we can find out more about the exact temperature of the core – and what it’s made of.
Mysterious chemistry
Traditionally, we have two ways to figure out what the core is made of: meteorites and seismology. By examining the chemistry of meteorites – which are thought to be pieces of planets that never formed, or pieces of the cores of destroyed Earth-like planets – we can get an idea of what our core could be made of.
The problem is that this only gives us a rough idea. Meteorites show us that the core should be made of iron and nickel, and maybe a few percent of silicon or sulphur, but it’s difficult to be more specific than this.
Seismology, on the other hand, is far more specific. When the sound waves from earthquakes travel through the planet, they speed up and slow down depending on what materials they pass through. By comparing the travel time of these waves, from earthquake to seismometer, with how fast waves travel through minerals and metals in experiments, we can get an idea of what the interior of the Earth is made of.
It turns out these travel times require that the Earth’s core is about 10% less dense than pure iron, and that the liquid outer core is denser than the solid inner core. Only some known chemistry of the core can explain these properties.
But even among a small selection of possible constituents, the potential melting temperatures vary by hundreds of degrees – leaving us none the wiser about the precise properties of the core.
A new constraint
In our new research, we’ve used mineral physics to study how the core might first have begun to freeze, discovering a new way to understand the chemistry of the core. And this approach appears to be even more specific than seismology and meteorites.

wikipedia, CC BY-SA
Research simulating how atoms in liquid metals come together to form solids has found that some alloys require more intense “supercooling” than others.
Supercooling is when a liquid is cooled below its melting temperature. The more intense the supercooling, the more often atoms will come together to form solids, making a liquid freeze faster. A water bottle in your freezer can be supercooled to -5°C for hours before freezing, whereas hail forms in minutes when water droplets are cooled to -30°C in clouds.
By exploring all possible melting temperatures of the core, we find that the most supercooled the core could have been is around 420°C below the melting temperature – any more than this and the inner core would be larger than seismology finds it to be. But pure iron requires an impossible ~1000°C of supercooling to freeze. If cooled this much, the entire core would have frozen, contrary to seismologists’ observations.
Adding silicon and sulphur, which both meteorites and seismology suggest could be present in the core, only make this problem worse – requiring even more supercooling.
Our new research explores the effect of carbon in the core. If 2.4% of the core’s mass was carbon, around 420°C of supercooling would be needed to begin freezing the inner core. This is the first time that freezing of the core has been shown to be possible. If the carbon content of the core was 3.8%, only 266°C of supercooling is needed. This is still a lot, but far more plausible.
This new finding shows that while seismology can narrow the possible chemistry of the core down to several different combinations of elements, many of these cannot explain the presence of the solid inner core at the centre of the planet.
The core cannot be made just of iron and carbon because the seismic properties of the core require at least one more element. Our research suggests it is more likely to contain a bit of oxygen and possibly silicon as well.
This marks a significant step toward understanding what the core is made of, how it started freezing, and how it has shaped our planet from the inside out.
![]()
Alfred Wilson-Spencer receives funding from the Natural Environment Research Council (NERC) grants NE/T000228/1 and NE/V010867/1. He works for the University of Leeds.