The development marks a milestone for a region that bears nearly 65 per cent of the global HIV burden and has long depended on imports of lifesaving antiretroviral medicines and testing kits. But that may be starting to change.
The human immunodeficiency virus (HIV) weakens the body’s immune system, reducing its ability to fight infections and certain cancers. Without timely intervention, it can progress to acquired immunodeficiency syndrome (AIDS), the most advanced stage of infection.
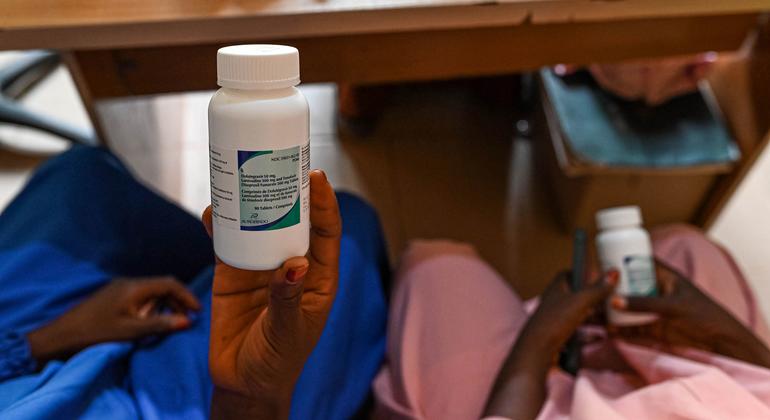
In 2023, Kenya-based pharmaceutical company Universal Corporation Ltd became the first African manufacturer to receive World Health Organization (WHO) prequalification to produce tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD) – a first-line antiretroviral therapy for HIV.
Now, in a major step forward, the Global Fund – a worldwide partnership financing HIV, tuberculosis and malaria responses – is procuring this locally produced HIV treatment for Mozambique, making it the first time African-manufactured TLD has been deployed through this channel.
“The procurement of the African-manufactured first-line HIV treatment by the Global Fund for Mozambique is a great milestone towards strengthening supply chain systems in Africa,” said Meg Doherty, Director of WHO’s Global HIV Programmes.
“This will contribute to better health outcomes for people living with HIV who need uninterrupted medicine supplies.”
Building regional capacity
WHO says the achievement is part of a broader push to bolster local production capacity and improve access to essential health technologies across Africa.
The UN agency has been partnering with countries, manufacturers and global health organizations – including the Global Fund and Unitaid – to expand quality-assured African manufacturing.
“Local production of quality-assured health products is an urgent priority,” said Rogerio Gaspar, WHO Director for Regulation and Prequalification.
“With every African manufacturer that meets WHO prequalification standards, we move closer to a more self-reliant, resilient and equitable health system.”
Progress, but structural gaps remain
Despite the milestone, WHO cautioned that production alone is not enough. To ensure long-term sustainability, the agency is calling for advanced market commitments, fair procurement policies and ongoing technical support.
WHO also points to diagnostics as a critical gap. With shifting donor funding, many countries are under pressure to maintain HIV testing programmes, which are the frontline of prevention and treatment.
In a related effort, Codix Bio, a Nigerian diagnostics company, recently received a sublicense to manufacture rapid diagnostic tests for HIV.
Locally produced HIV rapid tests will help increase affordability, and address supply chain vulnerabilities and delays
– WHO Director Meg Doherty
“Having locally produced HIV rapid tests will help increase affordability, and more broadly address supply chain vulnerabilities and delays in access to diagnostics,” said Dr. Doherty.
Sustaining impact amid funding strain
As part of its guidance, the UN health agency is also encouraging countries to adopt low-cost, WHO-prequalified rapid HIV tests, especially as the first test in national algorithms, which can significantly cut costs while maintaining service delivery.
While the latest update marks tangible progress, more action is needed.
“Locally manufactured TLD is a major step towards that goal,” WHO said, “but more action is needed.”