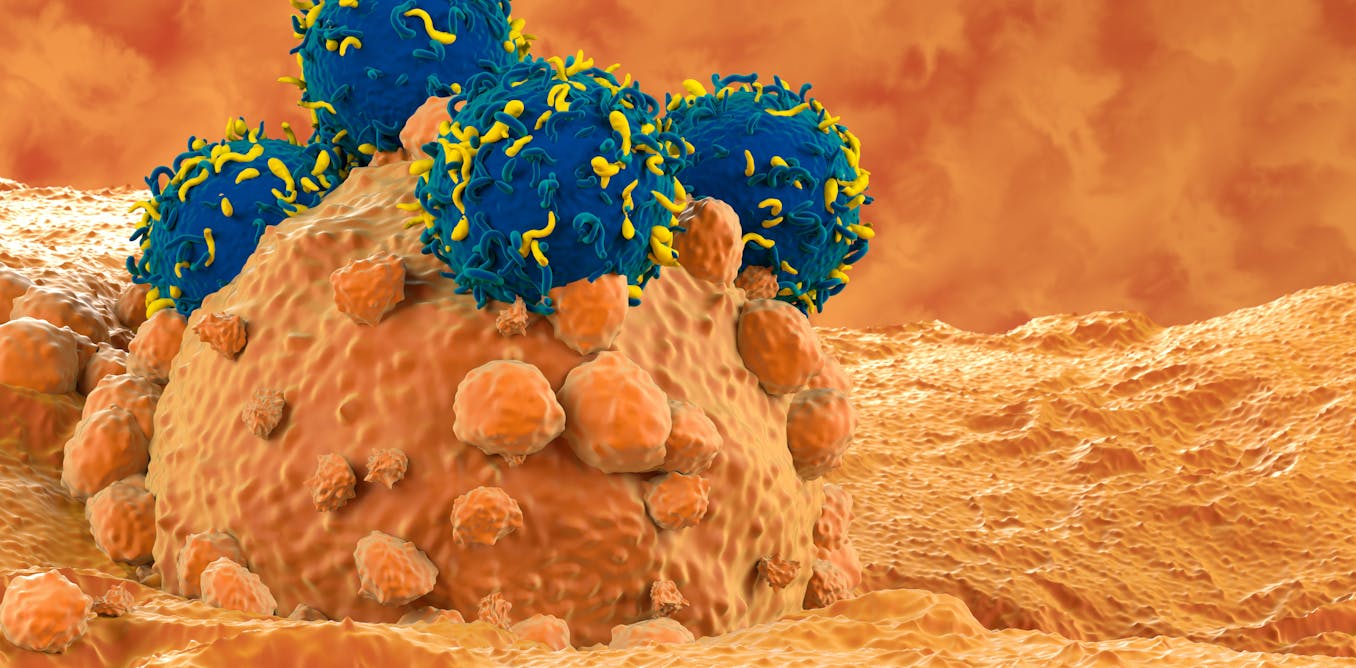
Personalised medicine is already a reality in clinical practice, and CAR-T cell therapy is one of its most promising tools. This innovative approach, which involves genetically modifying the cells of the immune system, is transforming the way we treat not only cancer, but also other diseases.
The CAR-T revolution is widely documented, with more than 1,000 clinical trials currently underway worldwide according to the ClinicalTrials platform. This reflects an enormous amount of scientific and medical interest.
To understand why it is of such interest, we first need to know what the function of a T-lymphocyte is. These cells belong to the immune system, and are responsible for finding and eliminating cells that are infected or have become abnormal, such as cancerous cells. We can think of them as the body’s police force: they recognise when something is wrong, and help to maintain the balance in our body.
“Latest generation” cells
CAR-T cells are, in essence, T-lymphocytes equipped with a next-generation membrane receptor: a structure specifically designed to recognise and attack tumour cells with a precision and sensitivity far superior to a normal T-cell. The difference is huge, like going from a mid-2000s Nokia phone to the latest iPhone.
Thanks to this modification, CAR-T cells can generate an immune response up to 1,000 times stronger than an ordinary lymphocyte. This makes it possible to eliminate cancer cells that do not respond to conventional treatments.
But what exactly is this alteration that proves so lethal to cancer cells? CAR-T cells are designed as “reconnaissance and attack” against cancer, and to do this, they have three main components. There is an external part that acts like a radar, identifying tumour cells; another part that passes through the cell membrane to transmit the signal inwards, and an internal part that triggers the T-cell response, activating its attack.
Although this basic design has already proven very powerful, researchers continue to improve it, both to make it even more effective and to prevent cells from becoming exhausted before they are able to kill off every last tumour cell.
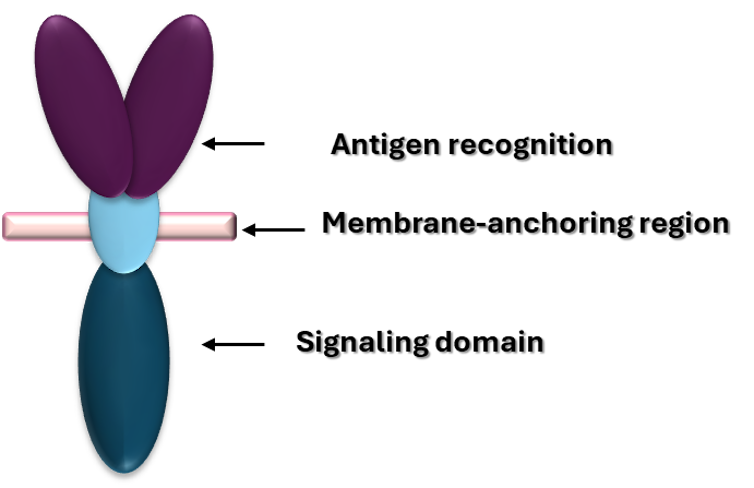
Therapeutic options for many patients have multiplied thanks both to the ability to develop these cells ex vivo – in the laboratory – and to advances in gene editing. To date, there are 7 CAR-T therapies which are authorised for use in patients.
Obstacles and limitations
Despite their efficacy, these therapies are not yet available to all patients due to their high cost and associated risks. This underlines the need to improve both their safety and accessibility.
Moreover, although CAR-T has been very successful in treating leukaemias and myelomas, its application in solid tumours remains a challenge. In these cases, the tumour microenvironment imposes additional barriers: T cells must pass through dense matrices that hinder their motility. The acidic environment also compromises their functionality, and nutrient shortages can lead to cellular exhaustion.
Such factors have so far limited the success of CAR-T therapies in solid tumours, but researchers continue to explore new ways to overcome these difficulties. One such method involves combining chemotherapy and radiotherapy to enable T-cells to reach tumour cells.
Combining CAR-T with immune checkpoint inhibitors – drugs that block certain signals used by tumour cells to avoid being attacked by the immune system – could also improve their effectiveness in such hostile environments.
However, it is not always possible to obtain lymphocytes from the patient, either due to limitations in quality or quantity or clinical conditions that make collection difficult. To address this, research is being done into allogeneic therapies that use “off the shelf” CAR-T cells. These use T-cells from healthy donors, and are stored ready for use when needed.
These methods not only reduce production times and costs, but also expand access to such treatments to a wider range of patients.
The biggest challenge: solid tumours
Although there are still several hurdles to overcome, the main challenge is the expanding the application of CAR-T cells to other cancers, especially solid tumours, which could benefit from these therapies. Examples include glioblastoma and metastatic ovarian cancer.
In the case of glioblastoma (a type of brain tumour), specific targets such as B7H3 or EGFRvIII are being investigated. These antigens are only expressed on tumour cells, improving the accuracy of these therapies.
Also under study are new delivery modes that cross the blood-brain barrier (a barrier that regulates the passage of what can and cannot reach the brain) and ways to modulate inflammation, which is critical in treating brain tumours.
For metastatic ovarian cancer, for which there is currently no curative treatment, CAR-Ts offer a promising alternative. Clinical trials have shown encouraging results by targeting certain new tumour markers.
Moreover, CAR-T therapies are not only being applied in oncology. They are also being investigated for use in the treatment of autoimmune diseases, with trials underway for diseases such as rheumatoid arthritis. This opens up new possibilities for the future use of these therapies.
Impact on the pharmaceutical industry
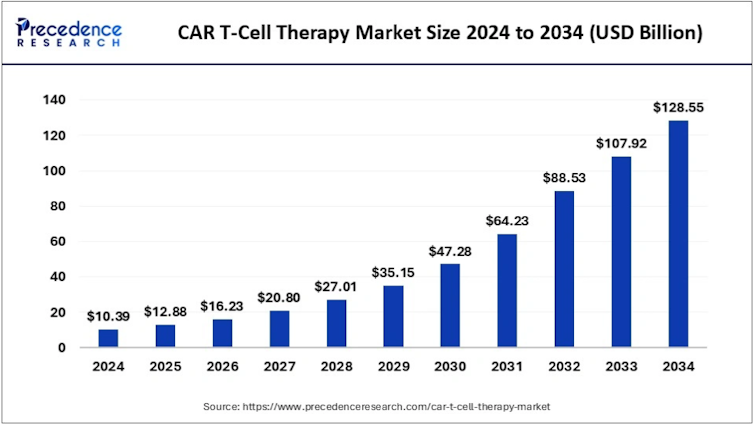
CAR-T’s advances, both consolidated and those on the horizon, have led the pharmaceutical industry to boost its investment in these therapies. Currently, the CAR-T cell therapeutics market is estimated to grow from $10.39 billion in 2024 to $128.55 billion in 2032.
The true potential of these therapies is yet to be discovered, and may lie in their effective application against solid tumours. However, one thing is for certain: what until recently seemed a futuristic concept is now a reality. Personalised medicine is here to stay, and CAR-T therapies are leading the way.