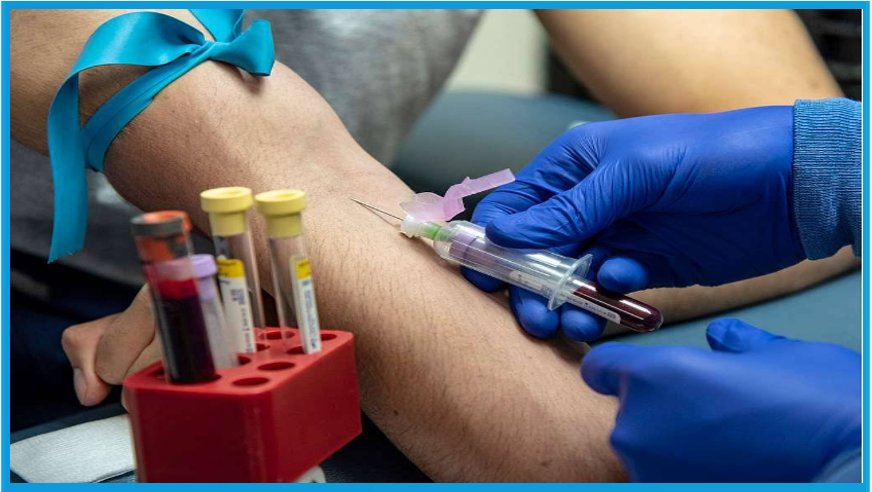
A new non-invasive blood test for liver cancer, developed by Roche Diagnostics, is now being trialled across hospitals within Manchester University NHS Foundation Trust (MFT), marking a major advancement in the early detection of hepatocellular carcinoma (HCC)—the most common type of liver cancer.
A Targeted Approach to a Deadly Disease
HCC is the third leading cause of cancer-related death in the UK, frequently developing in patients with pre-existing liver conditions such as cirrhosis. With roughly 3,000 new cases annually, only a third are diagnosed early enough for curative treatment, according to the British Liver Trust.
The newly launched Elecsys® GAAD test combines blood biomarkers with a patient’s age and sex to significantly improve the identification of early-stage HCC. It complements existing ultrasound-based surveillance, offering a more accurate and accessible alternative.
Promising Early Impact
Since its introduction in December 2023, the test has been administered to over 600 patients with cirrhosis at MFT clinics. It has already identified four early-stage cancers that would not have been detected using standard surveillance tools alone.
Speaking via an official statement published by MFT, Dr Varinder Athwal, Principal Investigator of the study, emphasised the potential of the test:
“Early results are very promising and show we are able to detect more cases of HCC at earlier, treatable stages which would have been missed by standard routine care. It truly has the potential to save lives.”
Dr Athwal also noted the regional significance, highlighting that Manchester has some of the UK’s highest rates of liver disease.
Toward a National Rollout
The study, supported by Imperial College London and health analytics firm Unity Insights, will continue until April 2025, aiming to recruit over 600 patients. Alongside clinical evaluation, the research will assess the economic viability of scaling the test across the NHS.
If successful, the Elecsys® GAAD test could become a critical part of the national liver cancer surveillance programme, potentially enabling more than 1,000 additional early-stage diagnoses per year in England alone.
Conclusion
This innovative screening method represents a tangible step toward improving liver cancer survival rates by catching the disease when curative treatments remain viable. As the UK continues to grapple with rising liver disease rates, advancements like this may play a pivotal role in transforming outcomes for thousands.
References:
- Manchester University NHS Foundation Trust: mft.nhs.uk
- British Liver Trust: britishlivertrust.org.uk
- Roche Diagnostics: diagnostics.roche.com
Blood Test Picture by Defense Visual Information Distribution Service